What Change To The Following Mrna Strand Would Result In A Missense Mutation
Learning Objectives
- Compare point mutations and frameshift mutations
- Describe the differences between missense, nonsense, and silent mutations
- Describe the differences between calorie-free and dark repair
- Explain how different mutagens act
- Explicate why the Ames examination can be used to find carcinogens
- Analyze sequences of DNA and place examples of types of mutations
A mutation is a heritable change in the Deoxyribonucleic acid sequence of an organism. The resulting organism, chosen a mutant, may take a recognizable change in phenotype compared to the wild type, which is the phenotype nigh normally observed in nature. A change in the DNA sequence is conferred to mRNA through transcription, and may atomic number 82 to an altered amino acrid sequence in a protein on translation. Because proteins carry out the vast majority of cellular functions, a modify in amino acid sequence in a poly peptide may lead to an altered phenotype for the cell and organism.
Effects of Mutations on Deoxyribonucleic acid Sequence
There are several types of mutations that are classified according to how the Deoxyribonucleic acid molecule is altered. One blazon, called a point mutation, affects a unmarried base of operations and near ordinarily occurs when i base is substituted or replaced by another. Mutations also result from the addition of one or more bases, known equally an insertion, or the removal of one or more bases, known as a deletion.
Call up about It
- What blazon of a mutation occurs when a gene has two fewer nucleotides in its sequence?
Effects of Mutations on Protein Construction and Function
Point mutations may have a broad range of effects on poly peptide function (Figure one). As a consequence of the degeneracy of the genetic code, a point mutation will commonly effect in the same amino acrid being incorporated into the resulting polypeptide despite the sequence change. This modify would accept no effect on the protein's construction, and is thus called a silent mutation. A missense mutation results in a different amino acid being incorporated into the resulting polypeptide. The effect of a missense mutation depends on how chemically unlike the new amino acid is from the wild-blazon amino acrid. The location of the changed amino acid within the protein likewise is important. For example, if the changed amino acid is function of the enzyme's agile site, then the effect of the missense mutation may be significant. Many missense mutations outcome in proteins that are still functional, at least to some degree. Sometimes the effects of missense mutations may exist only apparent under certain ecology conditions; such missense mutations are chosen conditional mutations. Rarely, a missense mutation may be benign. Under the correct environmental conditions, this type of mutation may give the organism that harbors it a selective reward. Yet another blazon of point mutation, called a nonsense mutation, converts a codon encoding an amino acid (a sense codon) into a stop codon (a nonsense codon). Nonsense mutations result in the synthesis of proteins that are shorter than the wild type and typically non functional.
Deletions and insertions also cause various effects. Because codons are triplets of nucleotides, insertions or deletions in groups of three nucleotides may atomic number 82 to the insertion or deletion of ane or more amino acids and may not crusade significant effects on the resulting protein's functionality. However, frameshift mutations, caused by insertions or deletions of a number of nucleotides that are not a multiple of iii are extremely problematic because a shift in the reading frame results (Effigy 1). Because ribosomes read the mRNA in triplet codons, frameshift mutations can alter every amino acrid after the point of the mutation. The new reading frame may besides include a end codon before the end of the coding sequence. Consequently, proteins fabricated from genes containing frameshift mutations are nearly always nonfunctional.
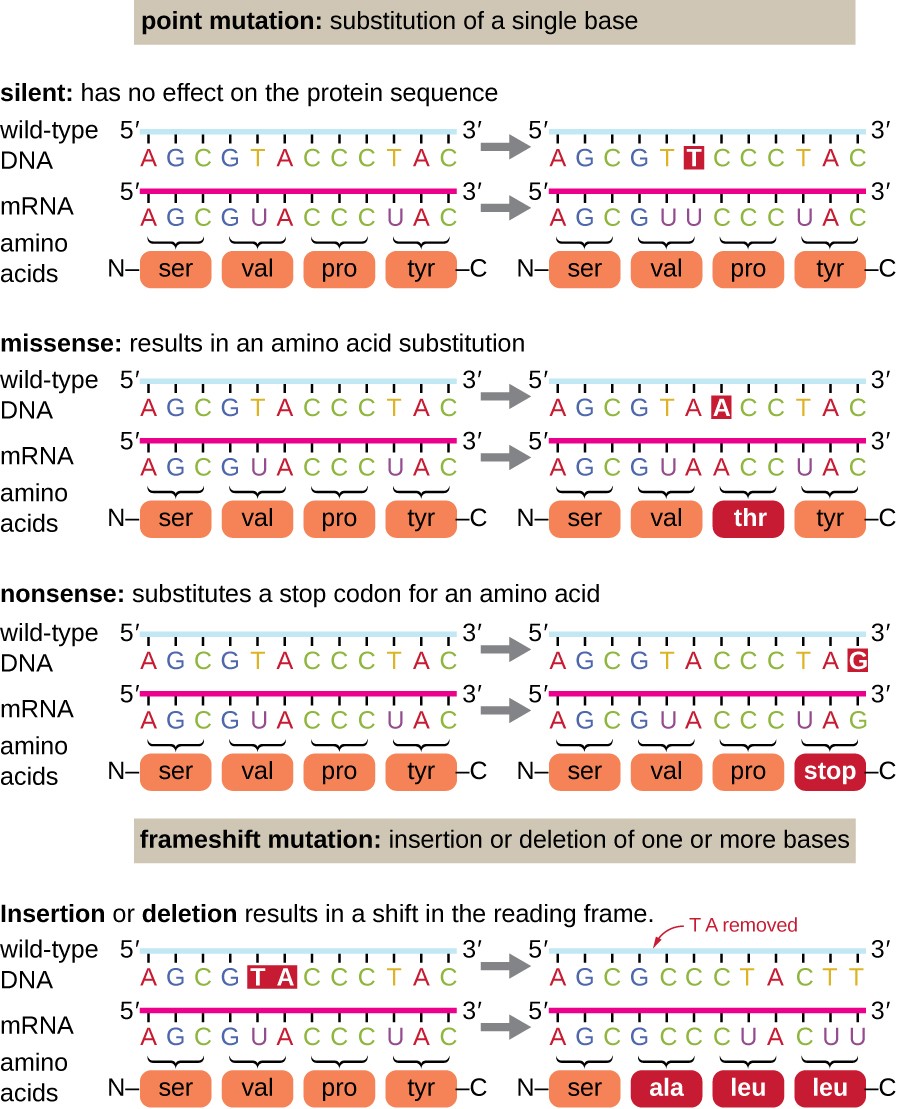
Effigy ane. Click for a larger prototype. Mutations tin atomic number 82 to changes in the poly peptide sequence encoded past the DNA.
Think about It
- What are the reasons a nucleotide alter in a gene for a protein might non have whatsoever effect on the phenotype of that gene?
- Is it possible for an insertion of 3 nucleotides together after the 5th nucleotide in a protein-coding gene to produce a poly peptide that is shorter than normal? How or how not?
A Beneficial Mutation
Since the get-go case of infection with human immunodeficiency virus (HIV) was reported in 1981, nearly 40 1000000 people have died from HIV infection,[1] the virus that causes acquired allowed deficiency syndrome (AIDS). The virus targets helper T cells that play a primal role in bridging the innate and adaptive immune response, infecting and killing cells usually involved in the trunk'south response to infection. There is no cure for HIV infection, just many drugs have been developed to ho-hum or block the progression of the virus. Although individuals effectually the world may be infected, the highest prevalence amongst people 15–49 years onetime is in sub-Saharan Africa, where nearly ane person in twenty is infected, accounting for greater than 70% of the infections worldwide[ii] (Figure 2). Unfortunately, this is as well a function of the world where prevention strategies and drugs to care for the infection are the most lacking.

Figure two. HIV is highly prevalent in sub-Saharan Africa, but its prevalence is quite low in some other parts of the world.
In recent years, scientific interest has been piqued past the discovery of a few individuals from northern Europe who are resistant to HIV infection. In 1998, American geneticist Stephen J. O'Brien at the National Institutes of Health (NIH) and colleagues published the results of their genetic analysis of more than iv,000 individuals. These indicated that many individuals of Eurasian descent (up to xiv% in some indigenous groups) have a deletion mutation, called CCR5-delta 32, in the gene encoding CCR5. CCR5 is a coreceptor found on the surface of T cells that is necessary for many strains of the virus to enter the host cell. The mutation leads to the production of a receptor to which HIV cannot finer bind and thus blocks viral entry. People homozygous for this mutation accept profoundly reduced susceptibility to HIV infection, and those who are heterozygous have some protection from infection too.
It is not clear why people of northern European descent, specifically, carry this mutation, just its prevalence seems to be highest in northern Europe and steadily decreases in populations as one moves southward. Inquiry indicates that the mutation has been nowadays since before HIV appeared and may take been selected for in European populations as a event of exposure to the plague or smallpox. This mutation may protect individuals from plague (caused by the bacterium Yersinia pestis) and smallpox (caused by the variola virus) because this receptor may also exist involved in these diseases. The age of this mutation is a matter of fence, but estimates advise it appeared between 1875 years to 225 years agone, and may have been spread from Northern Europe through Viking invasions.
This exciting finding has led to new avenues in HIV research, including looking for drugs to block CCR5 binding to HIV in individuals who lack the mutation. Although DNA testing to determine which individuals carry the CCR5-delta 32 mutation is possible, there are documented cases of individuals homozygous for the mutation contracting HIV. For this reason, DNA testing for the mutation is not widely recommended by public health officials so equally not to encourage risky behavior in those who carry the mutation. Nevertheless, inhibiting the binding of HIV to CCR5 continues to be a valid strategy for the evolution of drug therapies for those infected with HIV.
Causes of Mutations
Mistakes in the process of Dna replication can cause spontaneous mutations to occur. The error rate of Deoxyribonucleic acid polymerase is ane incorrect base per billion base pairs replicated. Exposure to mutagens can cause induced mutations, which are various types of chemical agents or radiation (Table ane). Exposure to a mutagen can increase the charge per unit of mutation more than than 1000-fold. Mutagens are often besides carcinogens, agents that cause cancer. However, whereas most all carcinogens are mutagenic, not all mutagens are necessarily carcinogens.
| Table one. A Summary of Mutagenic Agents | |||
|---|---|---|---|
| Mutagenic Agents | Mode of Action | Issue on Deoxyribonucleic acid | Resulting Type of Mutation |
| Nucleoside analogs | |||
| 2-aminopurine | Is inserted in place of A but base pairs with C | Converts AT to GC base pair | Point |
| 5-bromouracil | Is inserted in place of T but base pairs with G | Converts AT to GC base pair | Point |
| Nucleotide-modifying agent | |||
| Nitrous oxide | Deaminates C to U | Converts GC to AT base of operations pair | Indicate |
| Intercalating agents | |||
| Acridine orange, ethidium bromide, polycyclic aromatic hydrocarbons | Distorts double helix, creates unusual spacing betwixt nucleotides | Introduces pocket-size deletions and insertions | Frameshift |
| Ionizing radiations | |||
| X-rays, γ-rays | Forms hydroxyl radicals | Causes single- and double-strand Dna breaks | Repair mechanisms may introduce mutations |
| Ten-rays, γ-rays | Modifies bases (east.k., deaminating C to U) | Converts GC to AT base of operations pair | Point |
| Nonionizing radiation | |||
| Ultraviolet | Forms pyrimidine (commonly thymine) dimers | Causes DNA replication errors | Frameshift or point |
Chemical Mutagens
Various types of chemical mutagens interact directly with DNA either by interim every bit nucleoside analogs or by modifying nucleotide bases. Chemicals called nucleoside analogs are structurally similar to normal nucleotide bases and can be incorporated into Deoxyribonucleic acid during replication (Figure 3). These base of operations analogs induce mutations considering they oftentimes have different base of operations-pairing rules than the bases they replace. Other chemical mutagens tin alter normal Dna bases, resulting in different base-pairing rules. For example, nitrous acid deaminates cytosine, converting it to uracil. Uracil then pairs with adenine in a subsequent round of replication, resulting in the conversion of a GC base pair to an AT base pair. Nitrous acid also deaminates adenine to hypoxanthine, which base of operations pairs with cytosine instead of thymine, resulting in the conversion of a TA base pair to a CG base of operations pair.

Effigy 3. Click for a larger image. (a) 2-aminopurine nucleoside (2AP) structurally is a nucleoside analog to adenine nucleoside, whereas 5-bromouracil (5BU) is a nucleoside analog to thymine nucleoside. 2AP base pairs with C, converting an AT base pair to a GC base of operations pair afterwards several rounds of replication. 5BU pairs with G, converting an AT base pair to a GC base pair after several rounds of replication. (b) Nitrous acid is a different type of chemical mutagen that modifies already existing nucleoside bases like C to produce U, which base of operations pairs with A. This chemical modification, as shown here, results in converting a CG base pair to a TA base of operations pair.
Chemical mutagens known every bit intercalating agents work differently. These molecules slide between the stacked nitrogenous bases of the DNA double helix, distorting the molecule and creating atypical spacing betwixt nucleotide base of operations pairs (Figure iv). As a result, during DNA replication, DNA polymerase may either skip replicating several nucleotides (creating a deletion) or insert actress nucleotides (creating an insertion). Either outcome may lead to a frameshift mutation. Combustion products like polycyclic aromatic hydrocarbons are particularly dangerous intercalating agents that tin lead to mutation-caused cancers. The intercalating agents ethidium bromide and acridine orange are commonly used in the laboratory to stain DNA for visualization and are potential mutagens.

Effigy 4. Intercalating agents, such as acridine, introduce singular spacing between base pairs, resulting in Dna polymerase introducing either a deletion or an insertion, leading to a potential frameshift mutation.
Radiations
Exposure to either ionizing or nonionizing radiation can each induce mutations in DNA, although by different mechanisms. Stiff ionizing radiation like X-rays and gamma rays can cause single- and double-stranded breaks in the Deoxyribonucleic acid courage through the formation of hydroxyl radicals on radiations exposure (Effigy 5). Ionizing radiation can also alter bases; for instance, the deamination of cytosine to uracil, analogous to the action of nitrous acrid.[three] Ionizing radiation exposure is used to kill microbes to sterilize medical devices and foods, because of its dramatic nonspecific effect in damaging Deoxyribonucleic acid, proteins, and other cellular components (see Using Physical Methods to Control Microorganisms).
Nonionizing radiation, similar ultraviolet low-cal, is not energetic enough to initiate these types of chemical changes. Notwithstanding, nonionizing radiations can induce dimer formation between two adjacent pyrimidine bases, commonly two thymines, within a nucleotide strand. During thymine dimer formation, the two adjacent thymines become covalently linked and, if left unrepaired, both Deoxyribonucleic acid replication and transcription are stalled at this point. Deoxyribonucleic acid polymerase may go on and replicate the dimer incorrectly, potentially leading to frameshift or point mutations.

Figure 5. (a) Ionizing radiations may pb to the formation of unmarried-stranded and double-stranded breaks in the carbohydrate-phosphate backbone of Dna, as well equally to the modification of bases (not shown). (b) Nonionizing radiation like ultraviolet light tin can lead to the formation of thymine dimers, which tin stall replication and transcription and introduce frameshift or signal mutations.
Think about It
- How does a base of operations analog innovate a mutation?
- How does an intercalating amanuensis introduce a mutation?
- What type of mutagen causes thymine dimers?
Deoxyribonucleic acid Repair
The process of DNA replication is highly accurate, only mistakes can occur spontaneously or exist induced by mutagens. Uncorrected mistakes can pb to serious consequences for the phenotype. Cells take adult several repair mechanisms to minimize the number of mutations that persist.
Proofreading
About of the mistakes introduced during DNA replication are promptly corrected by most Deoxyribonucleic acid polymerases through a function chosen proofreading. In proofreading, the DNA polymerase reads the newly added base of operations, ensuring that information technology is complementary to the corresponding base of operations in the template strand earlier adding the next one. If an incorrect base has been added, the enzyme makes a cut to release the wrong nucleotide and a new base is added.
Mismatch Repair
Some errors introduced during replication are corrected shortly afterwards the replication machinery has moved. This mechanism is called mismatch repair. The enzymes involved in this machinery recognize the incorrectly added nucleotide, excise information technology, and replace it with the right base. One example is the methyl-directed mismatch repair in E. coli . The DNA is hemimethylated. This means that the parental strand is methylated while the newly synthesized girl strand is not. It takes several minutes before the new strand is methylated. Proteins MutS, MutL, and MutH demark to the hemimethylated site where the incorrect nucleotide is found. MutH cuts the nonmethylated strand (the new strand). An exonuclease removes a portion of the strand (including the incorrect nucleotide). The gap formed is then filled in past Dna politico III and ligase.
Repair of Thymine Dimers
Because the production of thymine dimers is common (many organisms cannot avoid ultraviolet light), mechanisms take evolved to repair these lesions. In nucleotide excision repair (also called dark repair), enzymes remove the pyrimidine dimer and replace it with the correct nucleotides (Figure 6). In E. coli, the DNA is scanned by an enzyme circuitous. If a distortion in the double helix is institute that was introduced by the pyrimidine dimer, the enzyme circuitous cuts the carbohydrate-phosphate backbone several bases upstream and downstream of the dimer, and the segment of DNA between these ii cuts is then enzymatically removed. DNA political leader I replaces the missing nucleotides with the correct ones and DNA ligase seals the gap in the sugar-phosphate backbone.
The direct repair (also chosen calorie-free repair) of thymine dimers occurs through the process of photoreactivation in the presence of visible light. An enzyme called photolyase recognizes the baloney in the DNA helix caused by the thymine dimer and binds to the dimer. Then, in the presence of visible low-cal, the photolyase enzyme changes conformation and breaks apart the thymine dimer, assuasive the thymines to once more correctly base of operations pair with the adenines on the complementary strand. Photoreactivation appears to be present in all organisms, with the exception of placental mammals, including humans. Photoreactivation is particularly important for organisms chronically exposed to ultraviolet radiation, similar plants, photosynthetic leaner, algae, and corals, to forbid the aggregating of mutations acquired by thymine dimer formation.

Figure 6. Click for a larger paradigm. Leaner accept 2 mechanisms for repairing thymine dimers. (a) In nucleotide excision repair, an enzyme complex recognizes the distortion in the DNA complex around the thymine dimer and cuts and removes the damaged Dna strand. The correct nucleotides are replaced by Dna pol I and the nucleotide strand is sealed past Dna ligase. (b) In photoreactivation, the enzyme photolyase binds to the thymine dimer and, in the presence of visible light, breaks apart the dimer, restoring the base pairing of the thymines with complementary adenines on the reverse Deoxyribonucleic acid strand.
Think about It
- During mismatch repair, how does the enzyme recognize which is the new and which is the sometime strand?
- How does an intercalating agent introduce a mutation?
- What type of mutation does photolyase repair?
Identifying Bacterial Mutants
Ane common technique used to identify bacterial mutants is called replica plating. This technique is used to discover nutritional mutants, called auxotrophs, which take a mutation in a gene encoding an enzyme in the biosynthesis pathway of a specific nutrient, such as an amino acid. Every bit a result, whereas wild-type cells retain the power to grow normally on a medium lacking the specific nutrient, auxotrophs are unable to grow on such a medium. During replica plating (Figure 7), a population of bacterial cells is mutagenized and so plated as private cells on a complex nutritionally complete plate and allowed to abound into colonies. Cells from these colonies are removed from this principal plate, often using sterile velvet. This velvet, containing cells, is then pressed in the same orientation onto plates of diverse media. At least one plate should also be nutritionally complete to ensure that cells are being properly transferred between the plates. The other plates lack specific nutrients, allowing the researcher to detect various auxotrophic mutants unable to produce specific nutrients. Cells from the respective colony on the nutritionally complete plate tin can exist used to recover the mutant for further study.

Effigy 7. Identification of auxotrophic mutants, like histidine auxotrophs, is done using replica plating. After mutagenesis, colonies that grow on nutritionally complete medium merely not on medium lacking histidine are identified as histidine auxotrophs.
Retrieve about Information technology
- Why are cells plated on a nutritionally complete plate in add-on to nutrient-deficient plates when looking for a mutant?
The Ames Test
The Ames test, developed by Bruce Ames (1928–) in the 1970s, is a method that uses bacteria for rapid, cheap screening of the carcinogenic potential of new chemic compounds. The exam measures the mutation rate associated with exposure to the chemical compound, which, if elevated, may indicate that exposure to this compound is associated with greater cancer risk. The Ames examination uses as the exam organism a strain of Salmonella typhimurium that is a histidine auxotroph, unable to synthesize its own histidine because of a mutation in an essential cistron required for its synthesis. Later on exposure to a potential mutagen, these leaner are plated onto a medium defective histidine, and the number of mutants regaining the power to synthesize histidine is recorded and compared with the number of such mutants that arise in the absence of the potential mutagen (Figure 8). Chemicals that are more mutagenic will bring about more mutants with restored histidine synthesis in the Ames test. Because many chemicals are not direct mutagenic but are metabolized to mutagenic forms by liver enzymes, rat liver extract is commonly included at the start of this experiment to mimic liver metabolism. Subsequently the Ames test is conducted, compounds identified as mutagenic are farther tested for their potential carcinogenic properties by using other models, including creature models similar mice and rats.
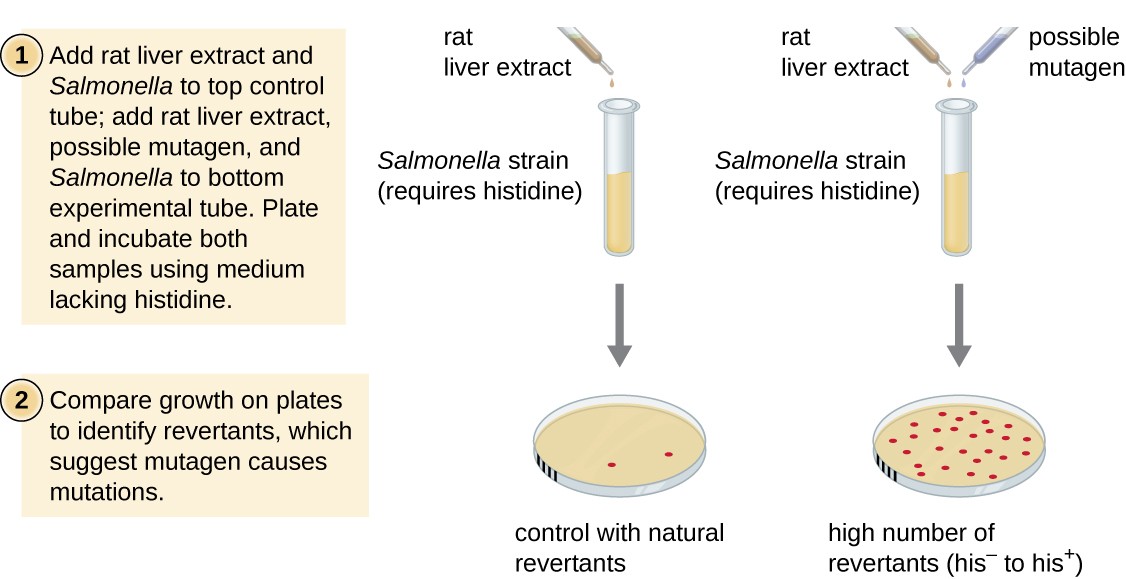
Effigy 8. The Ames test is used to identify mutagenic, potentially carcinogenic chemicals. A Salmonella histidine auxotroph is used as the exam strain, exposed to a potential mutagen/carcinogen. The number of reversion mutants capable of growing in the absence of supplied histidine is counted and compared with the number of natural reversion mutants that arise in the absence of the potential mutagen.
Think about It
- What mutation is used equally an indicator of mutation rate in the Ames test?
- Why can the Ames test work as a test for carcinogenicity?
Key Concepts and Summary
- A mutation is a heritable modify in Deoxyribonucleic acid. A mutation may lead to a change in the amino-acid sequence of a protein, possibly affecting its function.
- A point mutation affects a single base of operations pair. A point mutation may cause a silent mutation if the mRNA codon codes for the aforementioned amino acid, a missense mutation if the mRNA codon codes for a dissimilar amino acid, or a nonsense mutation if the mRNA codon becomes a finish codon.
- Missense mutations may retain part, depending on the chemistry of the new amino acid and its location in the protein. Nonsense mutations produce truncated and frequently nonfunctional proteins.
- A frameshift mutation results from an insertion or deletion of a number of nucleotides that is non a multiple of 3. The change in reading frame alters every amino acrid after the betoken of the mutation and results in a nonfunctional protein.
- Spontaneous mutations occur through DNA replication errors, whereas induced mutations occur through exposure to a mutagen.
- Mutagenic agents are frequently carcinogenic only non always. Withal, nearly all carcinogens are mutagenic.
- Chemical mutagens include base of operations analogs and chemicals that alter existing bases. In both cases, mutations are introduced after several rounds of DNA replication.
- Ionizing radiation, such as 10-rays and γ-rays, leads to breakage of the phosphodiester backbone of DNA and tin can besides chemically modify bases to alter their base-pairing rules.
- Nonionizing radiation similar ultraviolet light may introduce pyrimidine (thymine) dimers, which, during DNA replication and transcription, may introduce frameshift or betoken mutations.
- Cells have mechanisms to repair naturally occurring mutations. DNA polymerase has proofreading activity. Mismatch repair is a process to repair incorrectly incorporated bases after Dna replication has been completed.
- Pyrimidine dimers can also be repaired. In nucleotide excision repair (dark repair), enzymes recognize the distortion introduced by the pyrimidine dimer and supersede the damaged strand with the correct bases, using the undamaged DNA strand as a template. Leaner and other organisms may too utilize direct repair, in which the photolyase enzyme, in the presence of visible light, breaks apart the pyrimidines.
- Through comparison of growth on the complete plate and lack of growth on media lacking specific nutrients, specific loss-of-function mutants chosen auxotrophs can be identified.
- The Ames test is an inexpensive method that uses auxotrophic bacteria to measure mutagenicity of a chemic chemical compound. Mutagenicity is an indicator of carcinogenic potential.
Multiple Choice
Which of the following is a change in the sequence that leads to germination of a terminate codon?
- missense mutation
- nonsense mutation
- silent mutation
- deletion mutation
Show Answer
Answer b. A nonsense mutation is a alter in the sequence that leads to formation of a stop codon.
The formation of pyrimidine dimers results from which of the following?
- spontaneous errors past DNA polymerase
- exposure to gamma radiation
- exposure to ultraviolet radiation
- exposure to intercalating agents
Evidence Reply
Answer c. The formation of pyrimidine dimers results from exposure to ultraviolet radiation.
Which of the post-obit is an example of a frameshift mutation?
- a deletion of a codon
- missense mutation
- silent mutation
- deletion of i nucleotide
Bear witness Respond
Answer a. The deletion of i nucleotide is an case of a frameshift mutation.
Which of the post-obit is the blazon of DNA repair in which thymine dimers are directly cleaved down past the enzyme photolyase?
- direct repair
- nucleotide excision repair
- mismatch repair
- proofreading
Bear witness Respond
Answer a. In a directly repair, thymine dimers are straight cleaved down by the enzyme photolyase.
Which of the following regarding the Ames test is truthful?
- It is used to identify newly formed auxotrophic mutants.
- It is used to identify mutants with restored biosynthetic activeness.
- It is used to identify spontaneous mutants.
- It is used to identify mutants defective photoreactivation activity.
Evidence Respond
Answer b. Information technology is used to identify mutants with restored biosynthetic activity.
Fill up in the Blank
A chemic mutagen that is structurally similar to a nucleotide merely has different base-pairing rules is called a ________.
Testify Reply
A chemical mutagen that is structurally similar to a nucleotide but has unlike base-pairing rules is chosen a nucleoside analog.
The enzyme used in calorie-free repair to split up thymine dimers is chosen ________.
Show Answer
The enzyme used in light repair to carve up thymine dimers is chosen photolyase.
The phenotype of an organism that is about usually observed in nature is called the ________.
Testify Answer
The phenotype of an organism that is most commonly observed in nature is chosen the wild type.
True/Fake
Carcinogens are typically mutagenic.
Think most It
Why is information technology more than probable that insertions or deletions will exist more detrimental to a cell than point mutations?
Why practise you lot think the Ames exam is preferable to the use of animal models to screen chemical compounds for mutagenicity?
Disquisitional Thinking
Below are several DNA sequences that are mutated compared with the wild-type sequence: iii′-T A C T Chiliad A C T G A C G A T C-5′. Envision that each is a section of a DNA molecule that has separated in preparation for transcription, and so you are only seeing the template strand. Construct the complementary Deoxyribonucleic acid sequences (indicating 5′ and 3′ ends) for each mutated DNA sequence, and then transcribe (indicating 5′ and 3′ ends) the template strands, and translate the mRNA molecules using the genetic code, recording the resulting amino acid sequence (indicating the N and C termini). What type of mutation is each?
| Mutated DNA Template Strand #1: iii′-T A C T M T C T G A C G A T C-v′ | |
|---|---|
| Complementary DNA sequence: | |
| mRNA sequence transcribed from template: | |
| Amino acid sequence of peptide: | |
| Blazon of mutation: | |
| Mutated Dna Template Strand #2: three′-T A C G Yard A C T Thou A C G A T C-5′ | |
|---|---|
| Complementary DNA sequence: | |
| mRNA sequence transcribed from template: | |
| Amino acid sequence of peptide: | |
| Type of mutation: | |
| Mutated DNA Template Strand #3: 3′-T A C T G A C T Yard A C T A T C-v′ | |
|---|---|
| Complementary DNA sequence: | |
| mRNA sequence transcribed from template: | |
| Amino acrid sequence of peptide: | |
| Type of mutation: | |
| Mutated DNA Template Strand #4: 3′-T A C K A C T G A C T A T C-5′ | |
|---|---|
| Complementary DNA sequence: | |
| mRNA sequence transcribed from template: | |
| Amino acrid sequence of peptide: | |
| Blazon of mutation: | |
Source: https://courses.lumenlearning.com/microbiology/chapter/mutations/
Posted by: liddellpacconte.blogspot.com


0 Response to "What Change To The Following Mrna Strand Would Result In A Missense Mutation"
Post a Comment